CPMM study (2012)
Evaluation of an ultrasonic tonometer measuring carotid and femoral artery parameters
Date :
2012
State of progress :
Ended
Objective :
Evaluation of an ultrasonic tonometer measuring carotid
Partners :
ESA, MEDES, Verhaert
Context
When astronauts return to Earth after space flights of various durations, they frequently encounter symptoms such as dizziness when they change position; for example when moving from a sitting to a standing position, going from lying down to standing, or even when they remain in a standing position for long periods. This is known as orthostatic intolerance. It can be explained by a loss of the cardiovascular system’s ability to maintain a sufficient supply of oxygen to the brain. Symptoms can range from dizziness through to loss of consciousness.
Study objectives
In order to better assess these phenomena at arterial level under very specific conditions (microgravity, clinical simulation studies, parabolic flights, etc.), the European Space Agency decided to develop different devices aiming to better understand and analyse the physiological mechanisms involved in these symptoms. In this regard, and working closely with scientists from the University of Ghent, the Belgian company Verhaert is developing a device that combines the principles of tonometry and Doppler ultrasound to measure different parameters that characterise the physiological condition of the arteries. In particular, this device should measure blood pressure at various sites, arterial diameter, ejection time and pulse wave velocity, all in a completely non-invasive manner.
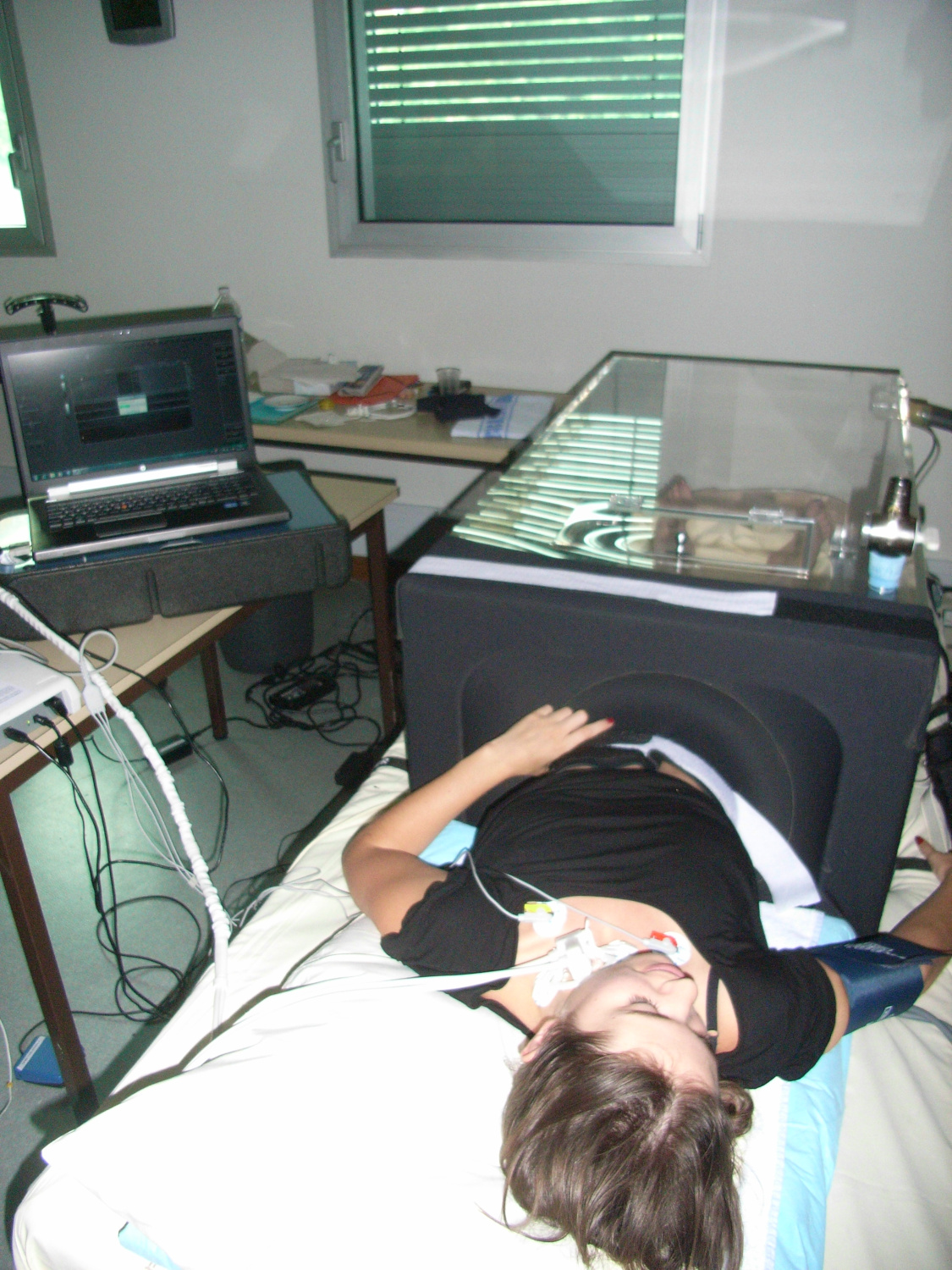
Apparatus tested : CPMM or tonometer and an ultrasound probe. This device records fine variations in pressure in relation to the sensor. It records movements linked to the heartbeat and the movement of blood in the vessels.
Implementation of the study
Fourteen male and female subjects aged from 20 to 40 years took part in this study.
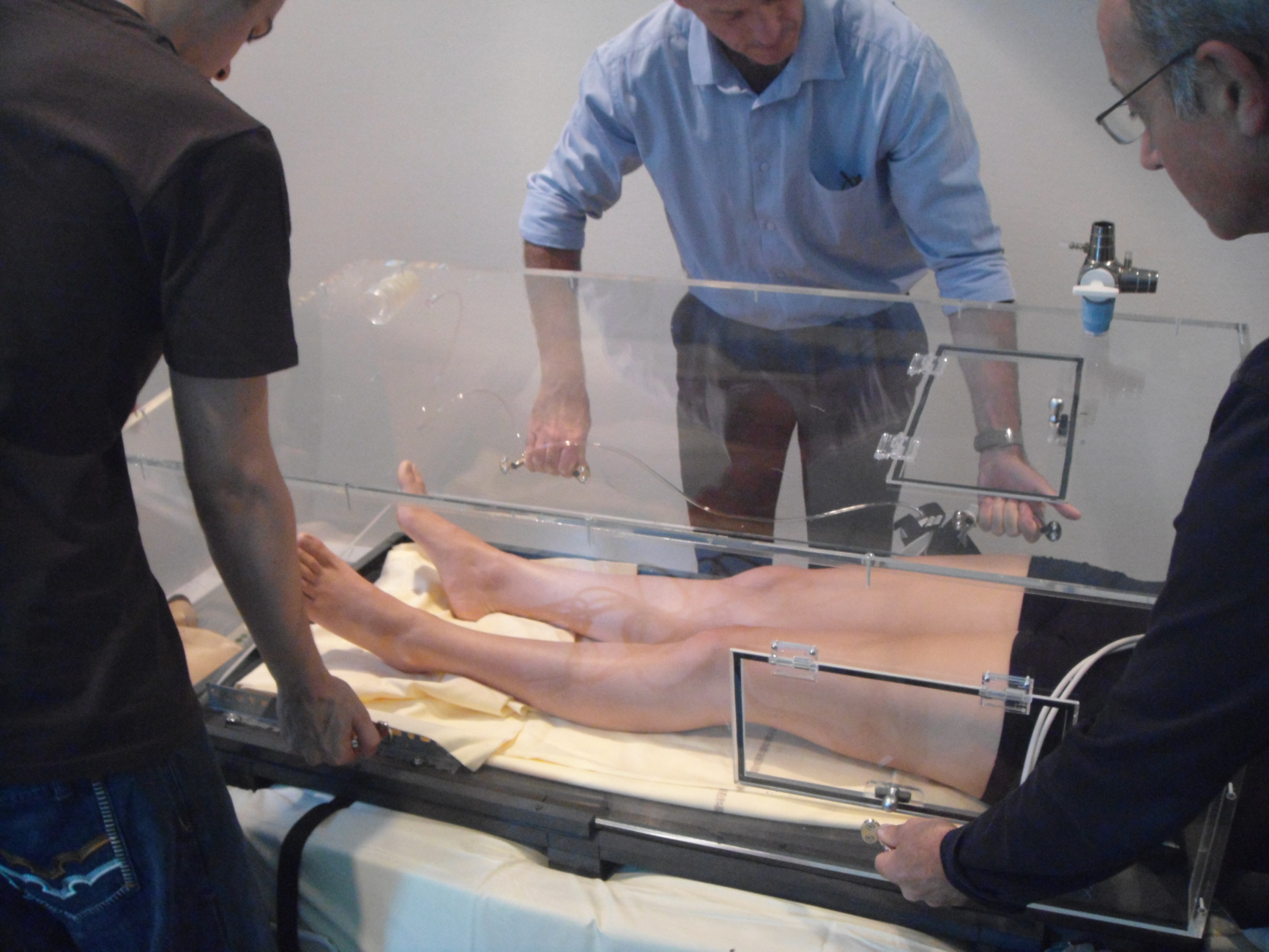
The subject lay on a kind of bed that can be mechanically lifted (a device called a tilt table). This tilt table is equipped with a “Low Body Negative Pressure” (LBNP) system, a large box in which the subject places his/her legs and pelvis. This system can then apply negative pressure (like a vacuum) to the lower part of the body. A neoprene skirt, similar to the one fitted to kayaks, ensures that the box is air-tight. A variety of equipment is then attached to the subject: ultrasound probes, tonometry sensors, Finapres monitor, Dynamap monitor, tilt table
Measurements from the LBNP during the tilt test are then recorded.
Nos actualités
Nos projets
Une question ?
Devenir volontaire ?
Nos études cliniques
Urgent ! Recrutement volontaires
